on
21++ Partition function boltzmann distribution ideas in 2021
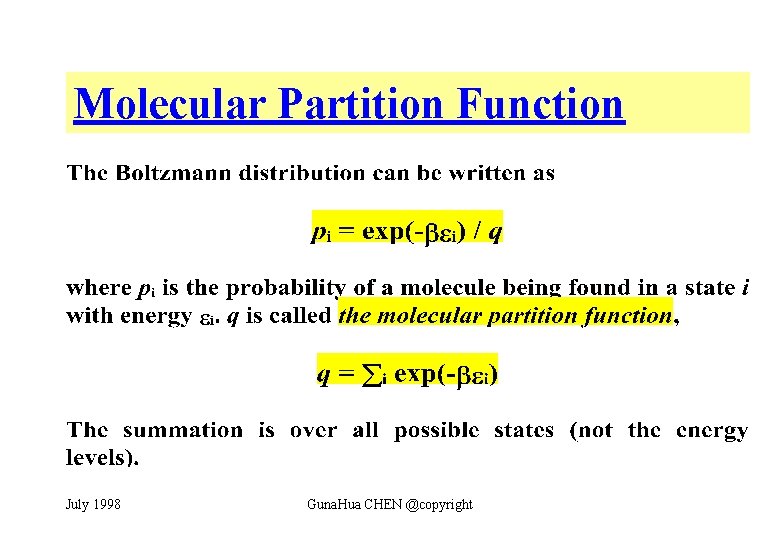
Partition Function Boltzmann Distribution. Thing called the partition function Z from which all thermodynamic quantities PEFS can be found. 15B4 shows schematically how p i varies with temperature. Uniform Ladder Because the partition function for the uniform ladder of energy levels is given by. Note that the normalisation of the Boltzmann distribution with the partition function occurswithinthewritingstep.
 Intermediate Physical Chemistry Driving Force Of Chemical Reactions From slidetodoc.com
Intermediate Physical Chemistry Driving Force Of Chemical Reactions From slidetodoc.com
Complete Courses Available for IIT JAM Physics CSIR NET Physical Science GATE Physics BHU JNU DU and Other MSc Physics Entrance Exams. Then the Boltzmann distribution for the populations in this system is. This partition function can be used for calculating the various thermodynamical properties of ensembles having independent systems obeying classical laws irrespective of whether the ensembles have distinguishable or. Beginalign n_i ebetaepsilon_i-mu. 22 Computing the Boltzmann Distribution of a Fictitious Harmonic Oscillator In this exercise you will apply the Boltzmann distribution. Larger the value of q larger the number of states which are available for the molecular system to occupy Figure PageIndex2.
Note that the normalisation of the Boltzmann distribution with the partition function occurswithinthewritingstep.
The partition function is a sum over states of course with the Boltzmann factor beta multiplying the energy in the exponent and is a number. Larger the value of q larger the number of states which are available for the molecular system to occupy Figure PageIndex2. Easy way to do this is to start y computing the Boltzmann distribution for just the x-axis velocities. 226 Calculating State Occupancy Finally. Endalign Aside from the fact that the single-state partition function you wrote down for the Maxwell-Boltzmann distribution yields this limit of the quantum ensemble average occupancies Im not sure at. If we have a model for a material for which we can calculate the partition function.
 Source: youtube.com
Source: youtube.com
1 Relative probability of two states. Boltzmann probabilities are - - - i i i i i i i i i i i i p Z p T S Z kT S k p p kT Z p kT Z ln 1 ln ln ln exp e e e e U TS kT Z S U T k Z ln ln - -. 22 Computing the Boltzmann Distribution of a Fictitious Harmonic Oscillator In this exercise you will apply the Boltzmann distribution. The problems are numbered to match the tags in the the lower left hand corner of the powerpoint slides. Endalign Aside from the fact that the single-state partition function you wrote down for the Maxwell-Boltzmann distribution yields this limit of the quantum ensemble average occupancies Im not sure at.
 Source: slideplayer.com
Source: slideplayer.com
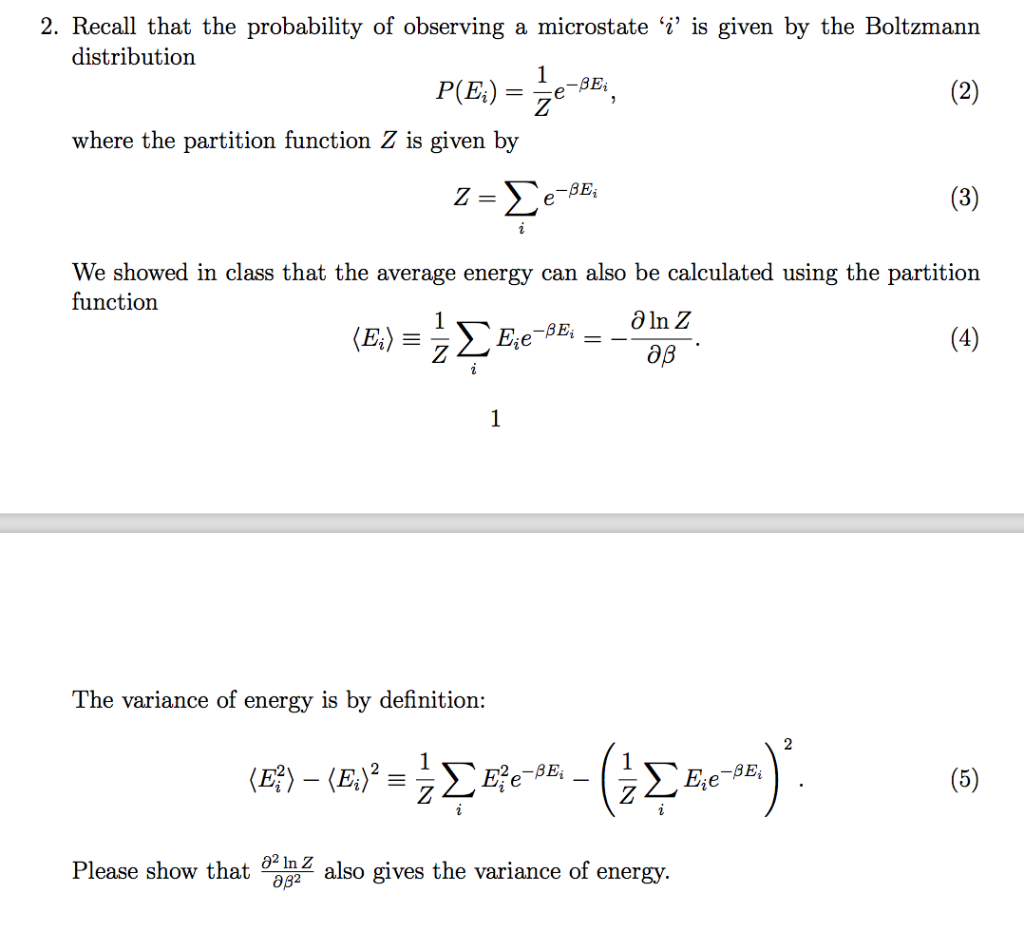
All thermodynamic quantities can be calculated from the partition function The Boltzmann factor and partition function are the two most important quantities for making statistical mechanical calculations. 22 Computing the Boltzmann Distribution of a Fictitious Harmonic Oscillator In this exercise you will apply the Boltzmann distribution. This partition function can be used for calculating the various thermodynamical properties of ensembles having independent systems obeying classical laws irrespective of whether the ensembles have distinguishable or. Boltzmann distribution partition function and internal energy L2 4449. Where the sum is over all the microstates of the system.
 Source: physics.stackexchange.com
Source: physics.stackexchange.com
Then the Boltzmann distribution for the populations in this system is. Recall F U-TS. Then the Boltzmann distribution for the populations in this system is. Boltzmann probabilities are - - - i i i i i i i i i i i i p Z p T S Z kT S k p p kT Z p kT Z ln 1 ln ln ln exp e e e e U TS kT Z S U T k Z ln ln - -. How can a constant be a function.
 Source: slidetodoc.com
Source: slidetodoc.com
Endalign Aside from the fact that the single-state partition function you wrote down for the Maxwell-Boltzmann distribution yields this limit of the quantum ensemble average occupancies Im not sure at. The Thermal Boltzman Distribution The Boltzmann distribution represents a thermally equilibrated most probable distribution over. 226 Calculating State Occupancy Finally. Complete Courses Available for IIT JAM Physics CSIR NET Physical Science GATE Physics BHU JNU DU and Other MSc Physics Entrance Exams. Larger the value of q larger the number of states which are available for the molecular system to occupy Figure PageIndex2.
 Source: chegg.com
Source: chegg.com
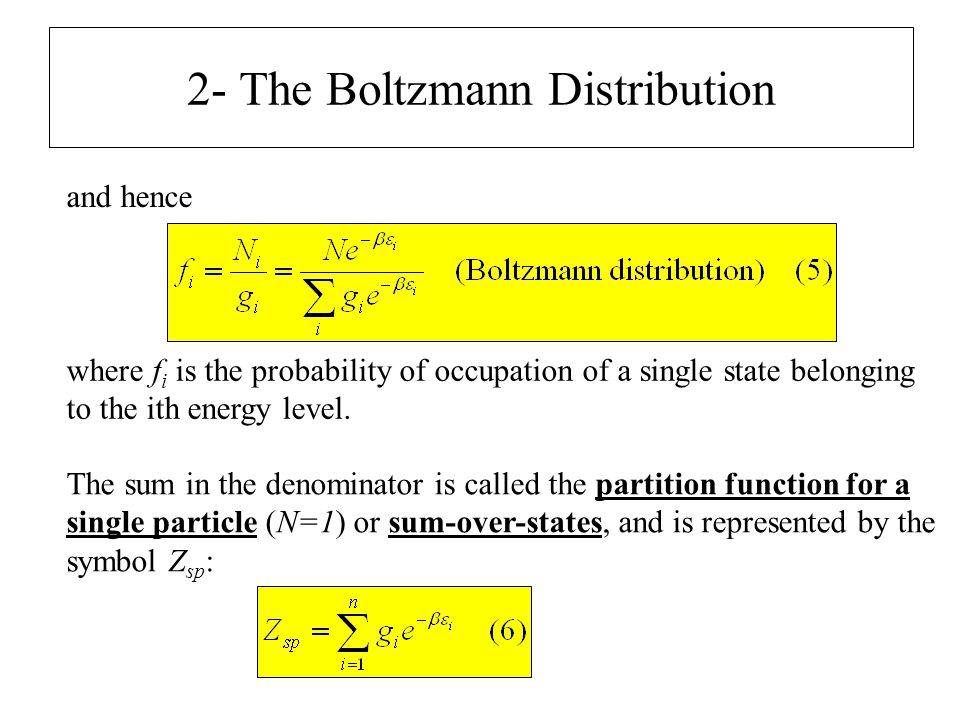
How can a constant be a function. Uniform Ladder Because the partition function for the uniform ladder of energy levels is given by. All thermodynamic quantities can be calculated from the partition function The Boltzmann factor and partition function are the two most important quantities for making statistical mechanical calculations. Boltzmann and Partition Function Examples These are the examples to be used along with the powerpoint lecture slides. The normalisation constant in the Boltzmann distribution is also called the partition function.
 Source: slideplayer.com
Source: slideplayer.com
Then the Boltzmann distribution for the populations in this system is. Easy way to do this is to start y computing the Boltzmann distribution for just the x-axis velocities. At very low T where q 1 only the lowest state is significantly populated. Partition function and the free energy The Boltzmann Gibbs equation allows us to determine the Free energy using the Boltzmann distribution to give us the probabilities p i. Complete Courses Available for IIT JAM Physics CSIR NET Physical Science GATE Physics BHU JNU DU and Other MSc Physics Entrance Exams.
 Source: slidetodoc.com
Source: slidetodoc.com
The Boltzmann factor is given by. At very low T where q 1 only the lowest state is significantly populated. To compute Boltzmann factor partition function must sum over all states of the particle. The Maxwell-Boltzmann distribution function is given by Occupation index. Note that the normalisation of the Boltzmann distribution with the partition function occurswithinthewritingstep.
 Source: studylib.net
Source: studylib.net
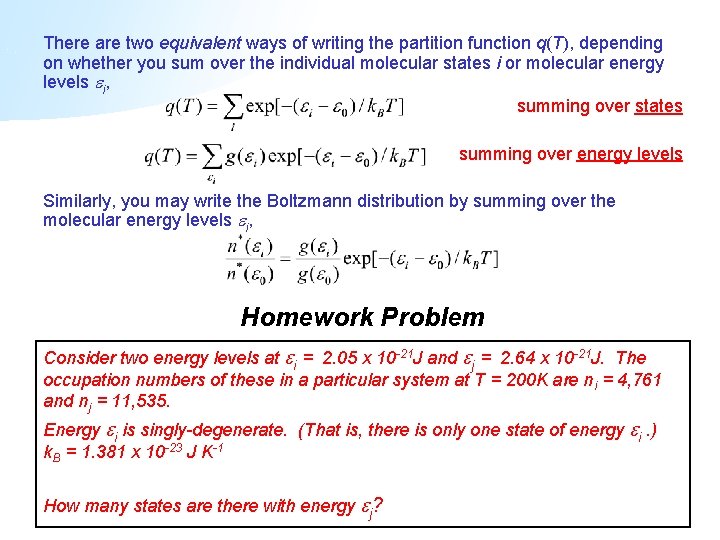
Partition function and the free energy The Boltzmann Gibbs equation allows us to determine the Free energy using the Boltzmann distribution to give us the probabilities p i. 15B4 shows schematically how p i varies with temperature. Larger the value of q larger the number of states which are available for the molecular system to occupy Figure PageIndex2. 226 Calculating State Occupancy Finally. The numbers of the examples are the in the EX-Boltz tags on the slides.
 Source: slideplayer.com
Source: slideplayer.com
Then the Boltzmann distribution for the populations in this system is. At the heart of the partition function lies the Boltz-mann distribution which gives the probability that a system in contact with a heat reservoir at a given temperature will have a given energy. 15B4 shows schematically how p i varies with temperature. Thing called the partition function Z from which all thermodynamic quantities PEFS can be found. Complete Courses Available for IIT JAM Physics CSIR NET Physical Science GATE Physics BHU JNU DU and Other MSc Physics Entrance Exams.
 Source: researchgate.net
Source: researchgate.net
At the heart of the partition function lies the Boltz-mann distribution which gives the probability that a system in contact with a heat reservoir at a given temperature will have a given energy. Partition function and the free energy The Boltzmann Gibbs equation allows us to determine the Free energy using the Boltzmann distribution to give us the probabilities p i. Complete Courses Available for IIT JAM Physics CSIR NET Physical Science GATE Physics BHU JNU DU and Other MSc Physics Entrance Exams. Boltzmann distribution partition function and internal energy L2 4449. The numbers of the examples are the in the EX-Boltz tags on the slides.
 Source: slideplayer.com
Source: slideplayer.com
Thing called the partition function Z from which all thermodynamic quantities PEFS can be found. 15B4 shows schematically how p i varies with temperature. The Boltzmann factor is used to approximate the fraction of particles in a large system. Complete Courses Available for IIT JAM Physics CSIR NET Physical Science GATE Physics BHU JNU DU and Other MSc Physics Entrance Exams. The problems are numbered to match the tags in the the lower left hand corner of the powerpoint slides.
 Source: slideplayer.com
Source: slideplayer.com
Then the Boltzmann distribution for the populations in this system is. Partition function and the free energy The Boltzmann Gibbs equation allows us to determine the Free energy using the Boltzmann distribution to give us the probabilities p i. 22 Computing the Boltzmann Distribution of a Fictitious Harmonic Oscillator In this exercise you will apply the Boltzmann distribution. Because particle has three velocities v x v y v z must sum over each of these when computing Q. If eg gi states have the same energy εi so the level is gi-fold degenerate where the sum is now over energy levels sets of states.
 Source: physics.stackexchange.com
Source: physics.stackexchange.com
Then the Boltzmann distribution for the populations in this system is. The Thermal Boltzman Distribution The Boltzmann distribution represents a thermally equilibrated most probable distribution over. When we take the classical limit of the quantum ensemble average occupancies we obtain the Maxwell-Boltzmann ensemble average occupancy. 1 Relative probability of two states. Then the Boltzmann distribution for the populations in this system is.
 Source: slideplayer.com
Source: slideplayer.com
At the heart of the partition function lies the Boltz-mann distribution which gives the probability that a system in contact with a heat reservoir at a given temperature will have a given energy. Thing called the partition function Z from which all thermodynamic quantities PEFS can be found. If eg gi states have the same energy εi so the level is gi-fold degenerate where the sum is now over energy levels sets of states. The Maxwell-Boltzmann distribution function is given by Occupation index. This partition function can be used for calculating the various thermodynamical properties of ensembles having independent systems obeying classical laws irrespective of whether the ensembles have distinguishable or.
 Source: chegg.com
Source: chegg.com
At very low T where q 1 only the lowest state is significantly populated. Beginalign n_i ebetaepsilon_i-mu. Thing called the partition function Z from which all thermodynamic quantities PEFS can be found. This partition function can be used for calculating the various thermodynamical properties of ensembles having independent systems obeying classical laws irrespective of whether the ensembles have distinguishable or. The Boltzmann factor is given by.
 Source: wikiwand.com
Source: wikiwand.com
At the heart of the partition function lies the Boltz-mann distribution which gives the probability that a system in contact with a heat reservoir at a given temperature will have a given energy. Partition function and the free energy The Boltzmann Gibbs equation allows us to determine the Free energy using the Boltzmann distribution to give us the probabilities p i. 1 Relative probability of two states. At very low T where q 1 only the lowest state is significantly populated. The Thermal Boltzman Distribution The Boltzmann distribution represents a thermally equilibrated most probable distribution over.
 Source: youtube.com
Source: youtube.com
At the heart of the partition function lies the Boltz-mann distribution which gives the probability that a system in contact with a heat reservoir at a given temperature will have a given energy. To compute Boltzmann factor partition function must sum over all states of the particle. 15B4 shows schematically how p i varies with temperature. Recall F U-TS. Easy way to do this is to start y computing the Boltzmann distribution for just the x-axis velocities.
 Source: slideplayer.com
Source: slideplayer.com
Because particle has three velocities v x v y v z must sum over each of these when computing Q. Beginalign n_i ebetaepsilon_i-mu. The normalisation constant in the Boltzmann distribution is also called the partition function. 162 The molecular partition function Boltzmann distribution where pi is the fraction of molecules in the state i pi niN and q is the molecular partition function. To compute Boltzmann factor partition function must sum over all states of the particle.